Recommendation Notice
The Importance of the QTPP for Cell Therapy Developers
The Quality Target Product Profile (QTPP) is a fundamental and key concept in the development of cell-based therapy products. We can define it as a forward-looking summary of the quality characteristics that a pharmaceutical product should ideally possess to guarantee the desired quality, considering safety and efficacy. In the Cell Therapy field, it’s well known that “The Product is The Process” but we often forget that “The QTPP defines the Product”.
WHY DEFINING THE QTPP IS CRUCIAL FOR YOUR PRODUCT’S SUCCESS
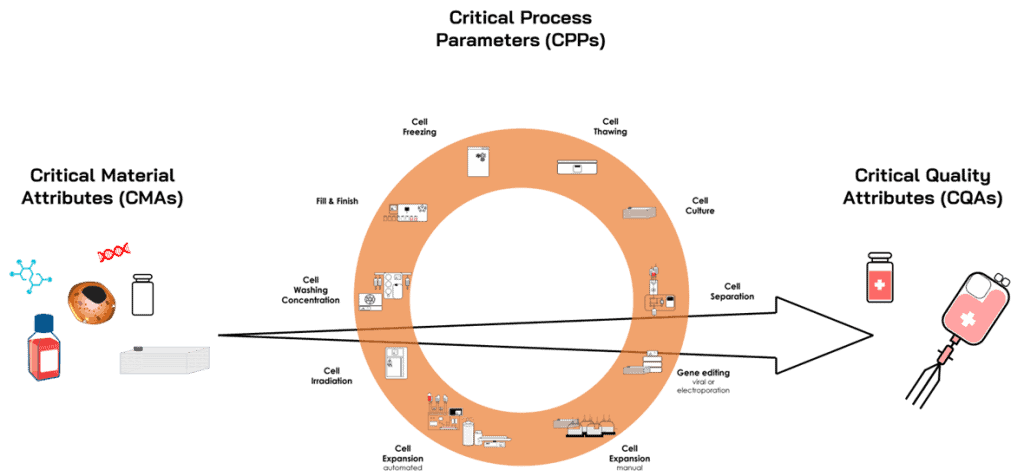
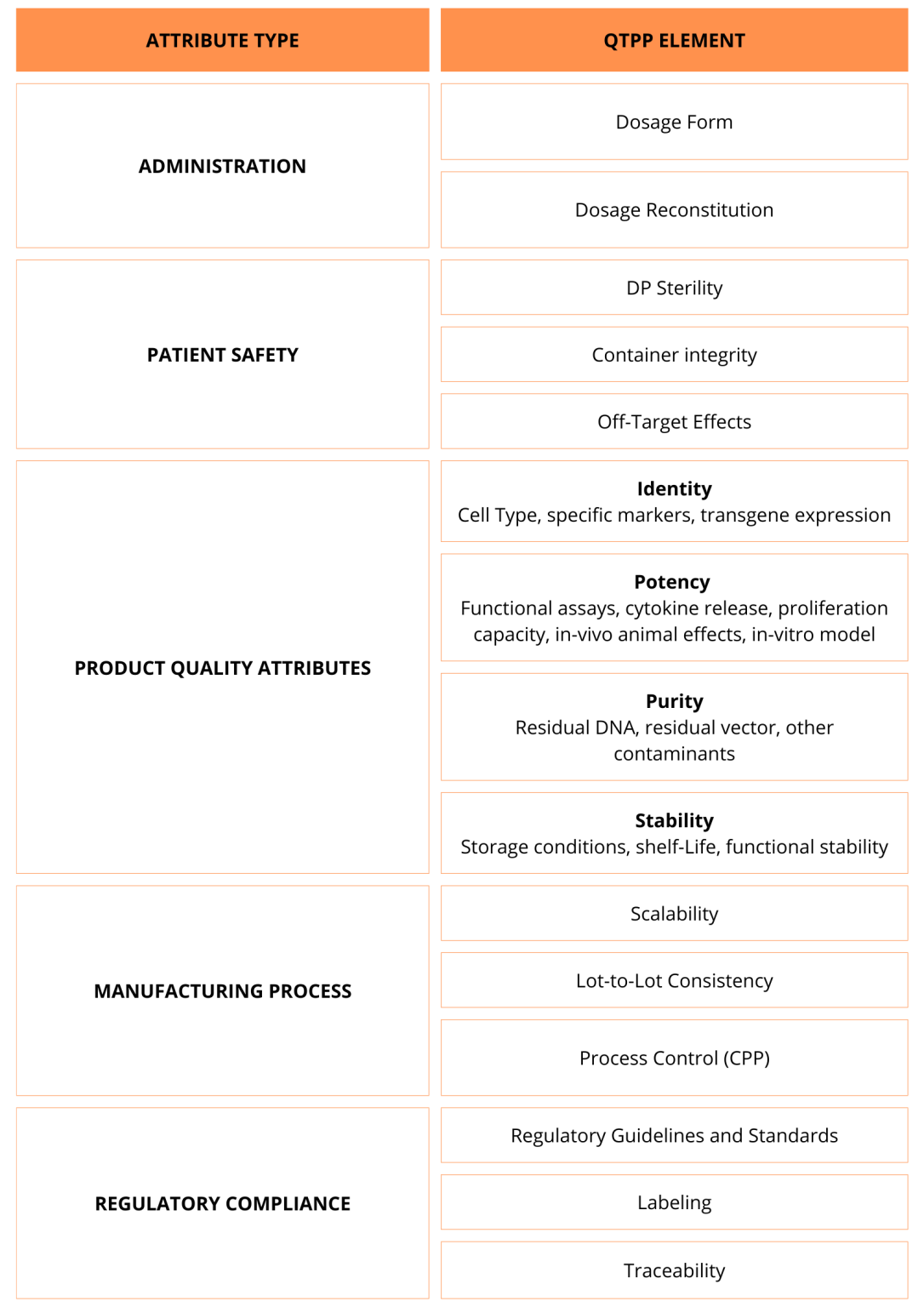
- Define Critical Quality Attributes (CQAs): The QTPP identifies the critical quality attributes (CQAs) of a cell therapy product—key physical, chemical, biological, and microbiological properties that must be controlled to ensure quality. These include the identity, potency, purity, viability, and safety of the therapeutic cells.
- Ensure Regulatory Compliance: Cell therapy products face strict regulatory requirements. The QTPP helps developers design products that comply, easing regulatory approvals and market access. Agencies like the FDA and EMA require comprehensive data on quality, safety, and efficacy, and a well-defined QTPP streamlines the preparation of submission documents.
- Support Quality by Design (QbD): The QTPP is central to the Quality by Design (QbD) approach, which focuses on building quality into the product from the start. This proactive method helps control variability in manufacturing, ensuring consistent product quality through product and process understanding, risk management, and statistical tools.
- Improve Patient Safety and Efficacy: By targeting key quality aspects, the QTPP ensures the cell therapy product is safe and effective for patients, especially in critically ill or vulnerable populations. This involves rigorous testing for contaminants, monitoring adverse reactions, and confirming the therapeutic cells function as intended.
- Facilitate Communication: The QTPP acts as a communication tool for multidisciplinary teams—scientists, engineers, clinicians, and regulatory professionals—ensuring a shared understanding of the product’s quality goals and development strategies. This fosters collaboration and alignment across departments, crucial for achieving product objectives.
- Guide Process Development: By setting clear quality goals, the QTPP guides the development of manufacturing processes, ensuring every step—from cell sourcing to final product formulation—meets the desired quality. It highlights all Critical Material Attributes (CMAs) and Critical Process Parameters (CPPs) that impact production, such as culture conditions, cell expansion, and storage, ensuring consistent product quality.
- Streamline Development and Production: A well-defined QTPP streamlines development by setting clear goals, minimizing trial-and-error, and leading to cost savings and shorter timelines. Early identification of critical attributes and process parameters lowers the risk of late-stage failures, ensuring a more efficient path to market.
CORE ELEMENTS OF AN EFFECTIVE QTPP
QTPP outlines the essential criteria needed to ensure the product’s therapeutic effectiveness and safety. It defines the key attributes required for a high-quality final product.
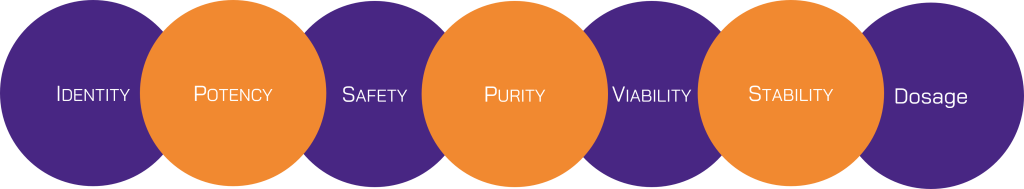
- Identity: Ensuring the cell type is correctly identified and characterized. This involves confirming the source, lineage, and specific markers of the therapeutic cells.
- Purity: Absence of contaminants, including unwanted cells or residual materials from the manufacturing process. This includes the removal of contamination cells, microbial contamination, and other impurities.
- Potency: The therapeutic effectiveness of the cell product. Potency assays are critical for demonstrating that the cell product performs its intended biological function.
- Viability: The percentage of live cells in the final product. High cell viability is essential for the therapeutic effectiveness and safety of the product.
- Safety: Ensuring no harmful effects, such as tumorigenicity or immunogenicity. This involves extensive preclinical testing to identify potential risks associated with cell therapy.
- Stability: Maintaining product quality over time during storage and transportation. Stability testing ensures that the product retains its efficacy and safety throughout its shelf life.
- Dosage: The concentration of cells or active ingredients in the product. Accurate quantification is necessary to ensure consistent dosing and therapeutic effects.
CELL EASY’S ROLE IN DEVELOPING YOUR QTPP TABLE
As a CDMO, we have witnessed numerous biotech companies face setbacks in their clinical trials because they overlooked the importance of establishing a robust QTPP. Neglecting this step often leads to unforeseen challenges in product development, affecting the safety, efficacy, and overall success of the therapy. At Cell Easy, our experienced team is equipped to assist you in creating your QTPP table by offering tailored insights and expertise at every stage of the process. Below is a non-exhaustive list of QTPP elements you should consider for your cell therapy, which we can help you address:
RECOMMENDATIONS FOR EARLY CLINICAL STAGE COMPANIES
- Engage with Regulatory Authorities Early: Early engagement with regulatory bodies can provide valuable guidance and ensure alignment with regulatory expectations. Consider seeking scientific advice or engaging in pre-IND (Investigational New Drug) meetings.
- Invest in Robust Analytical Methods: Developing reliable and sensitive analytical methods to assess CQAs is critical. These methods should be validated or at least qualified and capable of detecting subtle changes in product quality.
- Implement Comprehensive Risk Management: Use risk assessment tools such as Failure Modes and Effects Analysis (FMEA) to identify and mitigate potential risks throughout the product lifecycle.
- Focus on Scalable Manufacturing Processes: Ensure that the manufacturing processes are scalable and can be efficiently transitioned from early clinical stages to commercial production.
- Foster Cross-Functional Collaboration: Encourage collaboration between research, development, manufacturing, and regulatory teams to ensure a holistic approach to product development.
- Educate and Train Staff: Continuous education and training of staff on the principles of QTPP, Quality by Design (QbD), and regulatory requirements are essential for maintaining high standards of quality.
About Cell Easy
Cell-Easy is a science-driven CDMO, specializing in advanced cell therapies. Cell easy provides a wide range of services, including process and analytical development, GMP cell banking, GMP manufacturing, and comprehensive CMC/regulatory support for global biotech and pharma companies. Our scientific team, combined with a Quality by Design (QbD) approach, ensures seamless technology transfer and development of cell-based therapies in oncology, autoimmunity, and regenerative medicine. This includes T cells, NK cells, MSCs, macrophages, HSCs, and exosomes.
Explore our services at www.cell-easy.com or contact us at info@cell-easy.com.