Discover our Case Studies!

Maximize Working Cell Bank (WCB) Manufacturing Quantity while Controlling Batch Costs
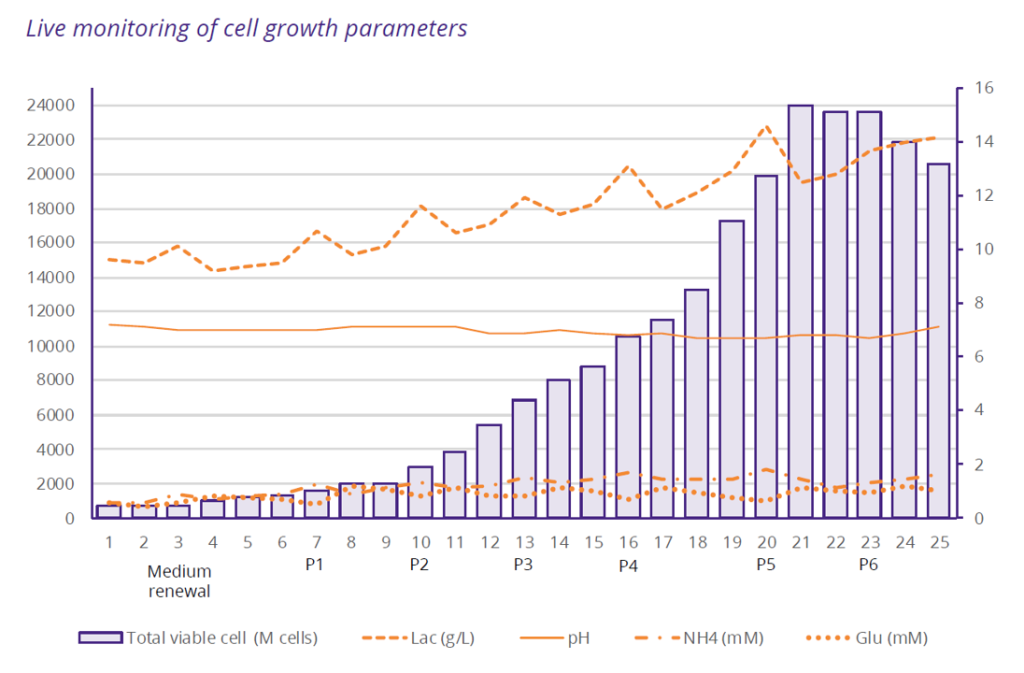
- Problem Statement: To develop a cell therapy product based on NK cells for a PhaseI clinical study, a European biotech company partnered with Cell-Easy to scale up and manufacture a GMP-compliant Working Cell Bank (WCB) from an immortalized feeder cell line. While the biotech had already established the 2 weeks process at lab-scale, the challenge was to scale it up and manufacture within a tight timeframe: 12 months to produce the first GMP batch of 50 billion cells.
- Our Approach: We have adopted a 3-stage strategy:
- Transfer and implementation of analytical methods.
- Control the scale up: Evaluation and testing of culture supports and conditions.
- Implementation in GMP environment.
- Outcomes: Thanks to our Quality by Design approach and the Cell Biology expertise of our multi-disciplinary teams, the WCB manufacturing process was successfully
scaled up — achieving a sixfold increase in cell production compared to the initial process — while maintaining the cell line’s quality attributes, adhering to the overall project timelines. In addition, the control of scale-up, batch-to-batch reproducibility and the increase in cell viability post-thawing have mechanically led to a 3-fold reduction in cost per billion cells manufactured.

An off-the-shelf MSC product derived from lab-scale autologous process
- Problem statement: Drawing on their scientific and medical expertise in the field of autologous MSCs, local researchers, professors and clinicians wanted to offer patients suffering from various autoimmune diseases an affordable and safe treatment.
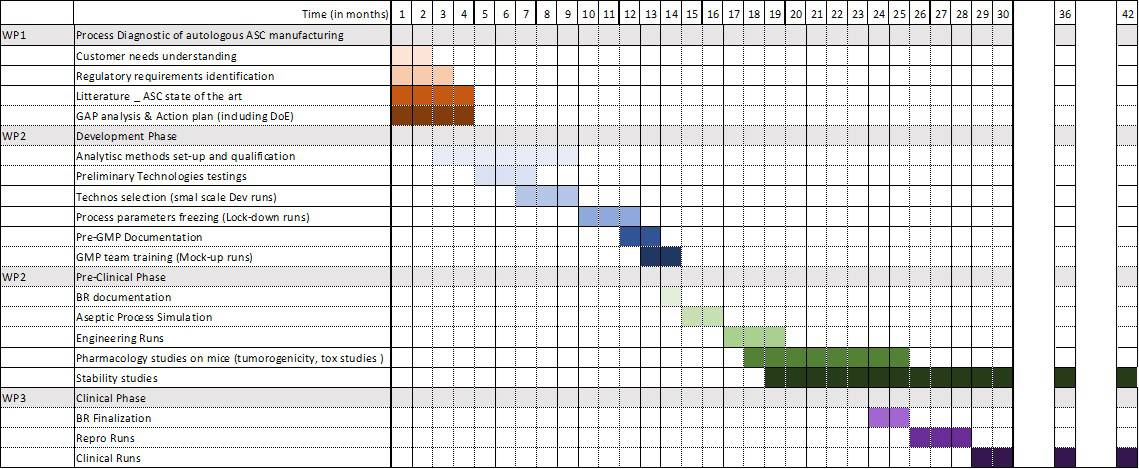
- Our Approach: In just 30 months, our dedicated teams have accomplished a significant milestone: the successful development, manufacturing, and validation of clinical-grade adipose-derived Mesenchymal Stem Cells (MSCs) based on a large-scale allogeneic process. These cells have received authorization from the French National Agency for Medicinal Products (ANSM) and are being injected in a range of Human clinical studies.
- Outcomes: Cell-Easy is offering R&D and GMP grade adipose-derived MSCs as:
Study model for Research Programs
- Drug Product in various Clinical Trials (systemic sclerosis, Crohn disease, Alzheimer disease, arthritis diseases,…)
- Starting material for the generation of GMP exosomes
- Cargo for various molecules and viruses